Great news! The mouse osteoblasts are ready to be seeded on the scaffolds Bryan Baker was kind enough to give me 400,000 hBMSC cells that he had cultured already. So I’m off and running on the second trial of this experiment! Here’s how I seeded the cells. Here’s the set up:  Next I needed to label the plates I would be using to hold the scaffolds, one plate for 18 scaffolds for the mC3T3-E1 cells, one plate of 18 scaffolds for the hBMSC-8001 cells and one plate of 6 scaffolds, 3 for each cell type to be run for 14 days.
Next I needed to label the plates I would be using to hold the scaffolds, one plate for 18 scaffolds for the mC3T3-E1 cells, one plate of 18 scaffolds for the hBMSC-8001 cells and one plate of 6 scaffolds, 3 for each cell type to be run for 14 days.  First I transferred the scaffolds to a sterile plate and then checked to make sure the curved side of the scaffold was facing down:
First I transferred the scaffolds to a sterile plate and then checked to make sure the curved side of the scaffold was facing down: 
 Next I need to add media to all the wells being careful to use the appropriate type of media for the different cell types:
Next I need to add media to all the wells being careful to use the appropriate type of media for the different cell types: 


 .
.  Next I used the vacuum to force the media into the cell holes:
Next I used the vacuum to force the media into the cell holes:  Then I was ready to remove 100 uL of media from each of the wells for the hBMSC-8001 cells and place it in the waste bucket:
Then I was ready to remove 100 uL of media from each of the wells for the hBMSC-8001 cells and place it in the waste bucket: 

 .Then I added 100 uL of the hBMSC-8001 cells to each of the 18 plus 3 wells with scaffolds
.Then I added 100 uL of the hBMSC-8001 cells to each of the 18 plus 3 wells with scaffolds 
 . Once the cells were plated it went into the incubator. Now needed to free the mC3T3-E1 cells that had adhered to the flask. First I transferred the media to the waste container:
. Once the cells were plated it went into the incubator. Now needed to free the mC3T3-E1 cells that had adhered to the flask. First I transferred the media to the waste container: 

 Then I added a Trypsin solution to the flask that helped free the cells from the flask
Then I added a Trypsin solution to the flask that helped free the cells from the flask 

 After 5 minutes I knocked the flask to free more cells then collected the solution:
After 5 minutes I knocked the flask to free more cells then collected the solution:  . I needed to centrifuge the solution to create a cell “pellet” so that I could count the number cells in the solution:
. I needed to centrifuge the solution to create a cell “pellet” so that I could count the number cells in the solution: 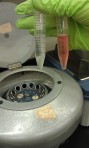
 Notice the white pellet at the bottom of the tube?
Notice the white pellet at the bottom of the tube?  I then poured off the supernatant (solution floating above the pellet):
I then poured off the supernatant (solution floating above the pellet): 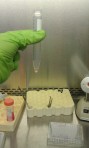 I then added 2 mL of media and mixed up the cells to determine the concentration using flow cytometry (see 6-26-12-seeding-the-cells-on-the-scaffolds for a discussion of how this works:
I then added 2 mL of media and mixed up the cells to determine the concentration using flow cytometry (see 6-26-12-seeding-the-cells-on-the-scaffolds for a discussion of how this works:  I found that there were 2,100,000 cells in my 2mL sample but I only needed concentration of 600,000 in 3 mL. I used the formula C1V1=C2V2 where C1= concentration 1, v1 = volume 1, c2= concentration 2, and v2= volume 2. Here’s where I used my algebra skills to plug in my known values to determine how much volume I needed to add to my media solution to get the correct concentration. 2,100,000 x ? = 600,000 x 3 mL
I found that there were 2,100,000 cells in my 2mL sample but I only needed concentration of 600,000 in 3 mL. I used the formula C1V1=C2V2 where C1= concentration 1, v1 = volume 1, c2= concentration 2, and v2= volume 2. Here’s where I used my algebra skills to plug in my known values to determine how much volume I needed to add to my media solution to get the correct concentration. 2,100,000 x ? = 600,000 x 3 mL
2,100,000 x ? = 1,800,000
? = 1,800,000/2,100,000
? = 0.857 mL . That means I needed to transfer 857 uL of cells solution to 2.143 mL of media to make final solution of 3 mL with a concentration of 200,000 cells. Then I needed to add 100 uL of this solution to each of the 21 scaffolds for the mC3T3-E1:  . These plates also got incubated for 24 hours as well. Up next Day 1 fluorescence imaging and freezing picogreen samples.
. These plates also got incubated for 24 hours as well. Up next Day 1 fluorescence imaging and freezing picogreen samples.



